Given the reaction:
4NH3(g) + 502(g) → 4NO(g) + 6H2O(g)
At constant pressure, how many liters...

Chemistry, 25.05.2021 19:20 issacbeecherpebpyl
Given the reaction:
4NH3(g) + 502(g) → 4NO(g) + 6H2O(g)
At constant pressure, how many liters of O2 (g)
would be required to produce 20 liters of NO (g)?
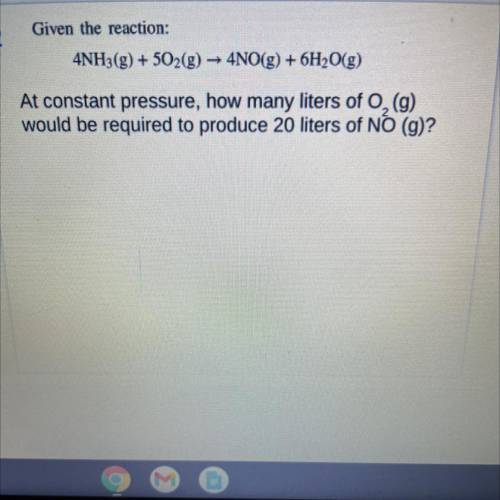

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1
You know the right answer?
Questions

Mathematics, 14.01.2021 22:20

Mathematics, 14.01.2021 22:20

Mathematics, 14.01.2021 22:20

Mathematics, 14.01.2021 22:20

Mathematics, 14.01.2021 22:20



Mathematics, 14.01.2021 22:20


Mathematics, 14.01.2021 22:20

Mathematics, 14.01.2021 22:20



Mathematics, 14.01.2021 22:20

Social Studies, 14.01.2021 22:20


Mathematics, 14.01.2021 22:20

Health, 14.01.2021 22:20

Mathematics, 14.01.2021 22:20



