
Chemistry, 25.05.2021 18:40 OkayLearn5522
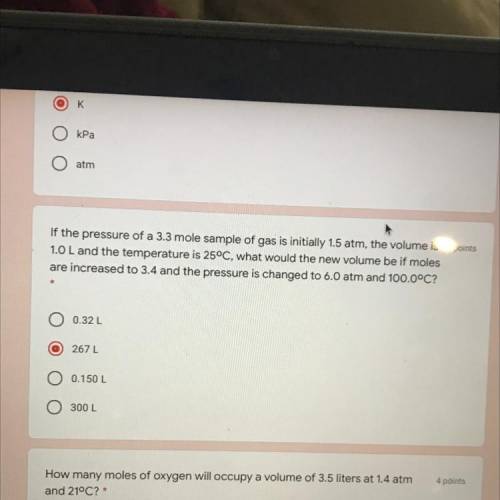
If the pressure of a 3.3 mole sample of gas is initially 1.5 atm, the volume is 4 points
1.0 L and the temperature is 25°C, what would the new volume be if moles
are increased to 3.4 and the pressure is changed to 6.0 atm and 100.0°C?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1


Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1
You know the right answer?
If the pressure of a 3.3 mole sample of gas is initially 1.5 atm, the volume is 4 points
1.0 L and...
Questions

Mathematics, 11.03.2021 07:20

Biology, 11.03.2021 07:20


Biology, 11.03.2021 07:20


English, 11.03.2021 07:20

History, 11.03.2021 07:20


Advanced Placement (AP), 11.03.2021 07:20

Mathematics, 11.03.2021 07:20

Mathematics, 11.03.2021 07:20

Engineering, 11.03.2021 07:20




Mathematics, 11.03.2021 07:20



Mathematics, 11.03.2021 07:20

Social Studies, 11.03.2021 07:20



