
Chemistry, 25.05.2021 18:00 Bucsan8688
A substance has a boiling point of 78 °C. Which of the following is true about the substance? (5 points) a It will also change from a gas to a solid at 78 °C while the gas loses energy. b It will also change from a gas to a solid at 78 °C while the gas gains energy. c It will also change from a gas to a liquid at 78 °C while the gas loses energy. d It will also change from a gas to a liquid at 78 °C while the gas gains energy.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3


Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2
You know the right answer?
A substance has a boiling point of 78 °C. Which of the following is true about the substance? (5 poi...
Questions







English, 18.09.2019 23:00

Arts, 18.09.2019 23:00



History, 18.09.2019 23:00


Mathematics, 18.09.2019 23:00

Biology, 18.09.2019 23:00

History, 18.09.2019 23:00



Mathematics, 18.09.2019 23:00


Mathematics, 18.09.2019 23:00

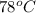 then it is true that it will also change from a gas to a liquid at 78 °C while the gas loses energy.
then it is true that it will also change from a gas to a liquid at 78 °C while the gas loses energy. 

