Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1


Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1

Chemistry, 23.06.2019 11:00
Acompound is isolated from the rind of lemons that is found to be 88.14% carbon and 11.86% hydrogen by mass how many grams of c and h?
Answers: 2
You know the right answer?
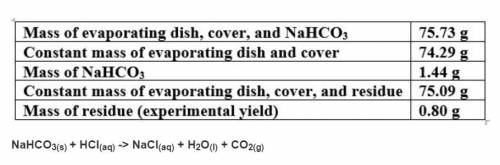
Based on a theoretical yield of 1.00 grams NaCl, calculate the mass in grams of hydrochloric acid yo...
Questions

Chemistry, 16.11.2020 18:10


Mathematics, 16.11.2020 18:10

Geography, 16.11.2020 18:10



Mathematics, 16.11.2020 18:10




Computers and Technology, 16.11.2020 18:10

History, 16.11.2020 18:10

Spanish, 16.11.2020 18:10


Health, 16.11.2020 18:10



Physics, 16.11.2020 18:10


History, 16.11.2020 18:10