Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3

Chemistry, 22.06.2019 23:30
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3

Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 2
You know the right answer?
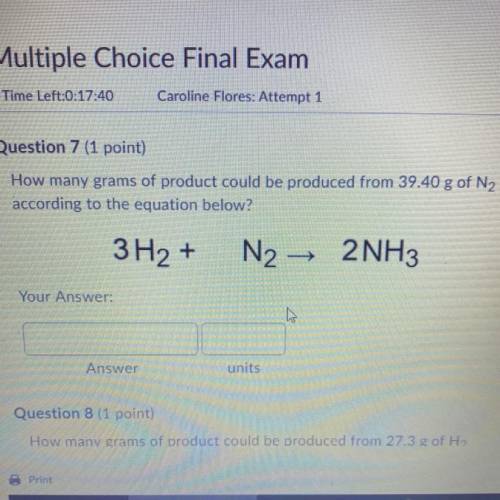
How many grams of product could be produced from 39.40 g of N2
according to the equation below?
Questions

Mathematics, 14.08.2021 03:00

Mathematics, 14.08.2021 03:00







Mathematics, 14.08.2021 03:00




Mathematics, 14.08.2021 03:00


Mathematics, 14.08.2021 03:00

Mathematics, 14.08.2021 03:00




Mathematics, 14.08.2021 03:00