
Chemistry, 24.05.2021 18:20 lizbethmillanvazquez
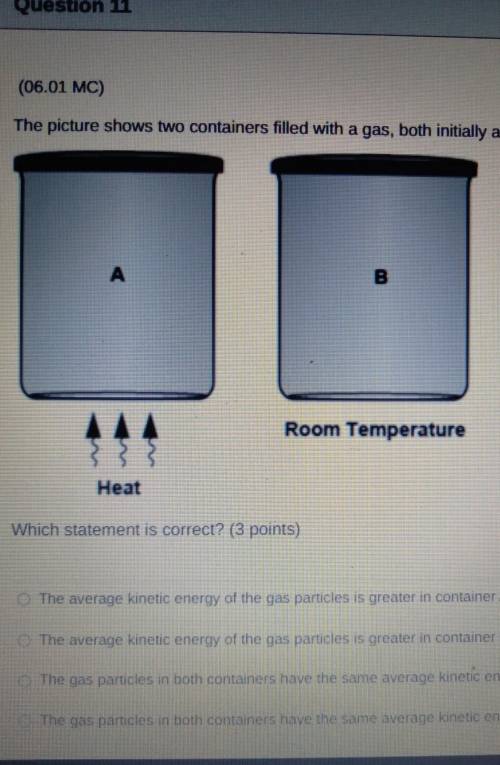
The picture shows two containers filled with a gas, both initially at room temperature.
A Heat
B Room Temperature
Which statement is correct?
The average kinetic energy of the gas particles is greater in container A because its particles move faster.
The average kinetic energy of the gas particles is greater in container B because it has a lower temperature.
The gas particles in both containers have the same average kinetic energy because they have the same volume.
The gas particles in both containers have the same average kinetic energy because they have equal number of particles

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
You know the right answer?
The picture shows two containers filled with a gas, both initially at room temperature.
A Heat
Questions





Social Studies, 02.01.2020 01:31

Social Studies, 02.01.2020 01:31

Mathematics, 02.01.2020 01:31




Advanced Placement (AP), 02.01.2020 01:31





Advanced Placement (AP), 02.01.2020 01:31


Mathematics, 02.01.2020 01:31




