Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1
You know the right answer?
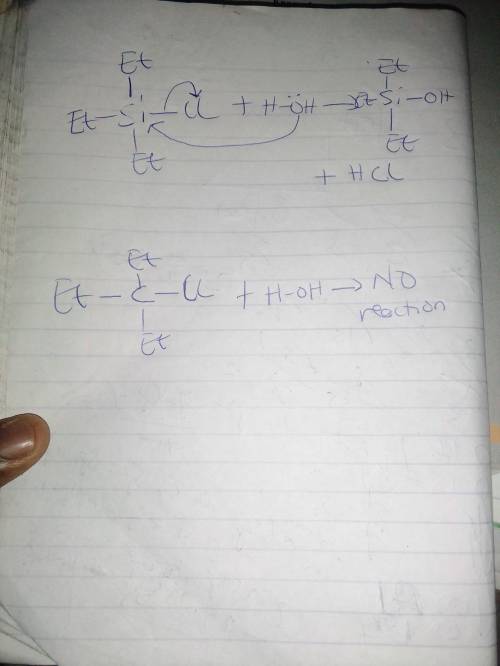
Whilst Et₃CCl is unreactive towards water even at elevated temperatures, Et₃SiCl is hydrolyzed rapid...
Questions

Biology, 23.11.2021 06:00




Mathematics, 23.11.2021 06:00

History, 23.11.2021 06:00

History, 23.11.2021 06:00

Mathematics, 23.11.2021 06:00






Mathematics, 23.11.2021 06:00



Chemistry, 23.11.2021 06:00

Mathematics, 23.11.2021 06:00