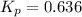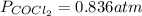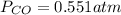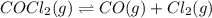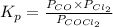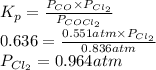The equilibrium constant, Kp, for the following reaction is 0.636 at 600K.
COCl2(g) <=> CO(g) + Cl2(g)
If an equilibrium mixture of the three gases in a 16.9 L container at 600K contains COCl2 at a pressure of 0.836 atm and CO at a pressure of 0.551 atm, the equilibrium partial pressure of Cl2 is atm.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which compounds have the empirical formula ch2o? a.c2h4o2 b.c3h6o3 c.ch2o2 d.c5h10o5 e.c6h12o6
Answers: 3

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3
You know the right answer?
The equilibrium constant, Kp, for the following reaction is 0.636 at 600K.
COCl2(g) <=> CO(g)...
Questions


History, 11.10.2019 14:30

Biology, 11.10.2019 14:30

Health, 11.10.2019 14:30


Mathematics, 11.10.2019 14:30

Computers and Technology, 11.10.2019 14:30


Business, 11.10.2019 14:30


Social Studies, 11.10.2019 14:30

Biology, 11.10.2019 14:30


History, 11.10.2019 14:30

History, 11.10.2019 14:30

Mathematics, 11.10.2019 14:30

Biology, 11.10.2019 14:30

Mathematics, 11.10.2019 14:30


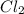 is 0.964 atm.
is 0.964 atm.