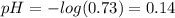Chemistry, 24.05.2021 09:10 alexkrol30083
A 5.00 mL sample of hydrochloric acid is titrated with 0.1293 M ammonia (a base). If the titration required 28.15 mL of ammonia, determine the following:
the original concentration of the acid
the original pH of the acid

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2
You know the right answer?
A 5.00 mL sample of hydrochloric acid is titrated with 0.1293 M ammonia (a base). If the titration r...
Questions

Spanish, 09.11.2020 19:10

Spanish, 09.11.2020 19:10

Mathematics, 09.11.2020 19:10

Chemistry, 09.11.2020 19:10

Mathematics, 09.11.2020 19:10


Mathematics, 09.11.2020 19:10

Physics, 09.11.2020 19:10

Mathematics, 09.11.2020 19:10


Mathematics, 09.11.2020 19:10

Mathematics, 09.11.2020 19:10

Chemistry, 09.11.2020 19:10

Mathematics, 09.11.2020 19:10


Biology, 09.11.2020 19:10

Mathematics, 09.11.2020 19:10

Mathematics, 09.11.2020 19:10



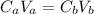
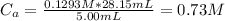
![pH = -log([H^{+}])](/tpl/images/1343/3808/0d4b9.png)