
PLEASE HELP!
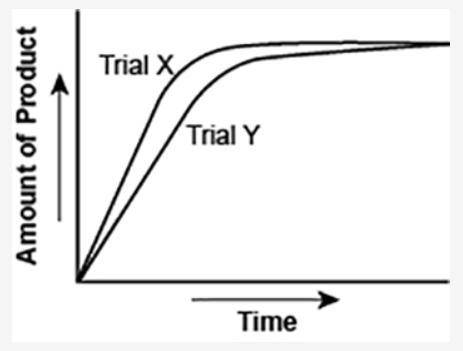
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different concentration of reactant was used during each trial, whereas the other factors were kept constant.
(I've attached the graph.)
Which of the following statements explains which trial has a lower concentration of the reactant?
a. Trial X, because the final volume of product formed is lower than Trial Y.
b. Trial X, because this reaction was initially fast and later stopped completely.
c. Trial Y, because the reaction was initially slow and later stopped completely.
d. Trial Y, because the volume of product formed per unit time is lower than Trial X.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1


Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3

Chemistry, 22.06.2019 19:20
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1
You know the right answer?
PLEASE HELP!
The graph shows the volume of a gaseous product formed during two trials of a reaction...
Questions

Mathematics, 03.08.2019 21:30

History, 03.08.2019 21:30

History, 03.08.2019 21:30




Biology, 03.08.2019 21:30

History, 03.08.2019 21:30



History, 03.08.2019 21:30

Mathematics, 03.08.2019 21:30

History, 03.08.2019 21:30

Biology, 03.08.2019 21:30

Biology, 03.08.2019 21:30


History, 03.08.2019 21:30


Biology, 03.08.2019 21:30

History, 03.08.2019 21:30



