
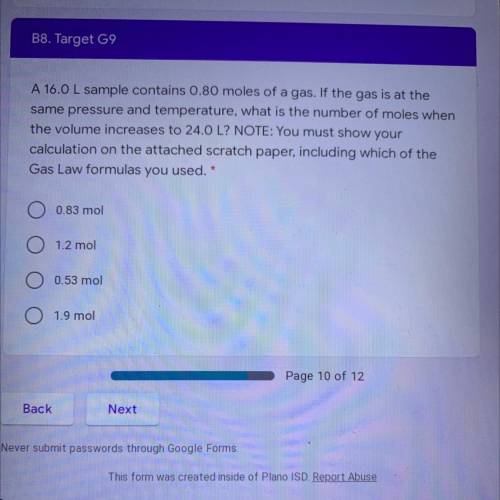
A 16.0 L sample contains 0.80 moles of a gas. If the gas is at the
same pressure and temperature, what is the number of moles when
the volume increases to 24.0 L? NOTE: You must show your
calculation on the attached scratch paper, including which of the
Gas Law formulas you used. *
A. 0.83 mol
B. 1.2 mol
C. 0.53 mol
D. 1.9 mol
(Please show your work)

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1

Chemistry, 22.06.2019 19:30
What is the area in square meters of 448 g ai foil that has a thickness of 23921 nm? the density is 2.70 g/cm
Answers: 3
You know the right answer?
A 16.0 L sample contains 0.80 moles of a gas. If the gas is at the
same pressure and temperature, w...
Questions

English, 29.10.2019 03:31


English, 29.10.2019 03:31





Mathematics, 29.10.2019 03:31




Mathematics, 29.10.2019 03:31


Arts, 29.10.2019 03:31

Mathematics, 29.10.2019 03:31




Mathematics, 29.10.2019 03:31

English, 29.10.2019 03:31

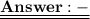
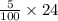 = 120/100= 12/10= 1.2 Mole ( Ans )
= 120/100= 12/10= 1.2 Mole ( Ans )

