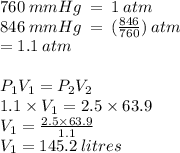Chemistry, 22.05.2021 16:00 carlosd21151
Nitrogen gas exerts a pressure of 846mmhg. When the pressure is changed to 2.5atm its volume is 63.9l. What was the original volume

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Complete this brønsted-lowry reaction placing each product by its appropriate label. hso4- + hcn
Answers: 1

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1
You know the right answer?
Nitrogen gas exerts a pressure of 846mmhg. When the pressure is changed to 2.5atm its volume is 63.9...
Questions



Health, 20.10.2020 20:01

Mathematics, 20.10.2020 20:01




Mathematics, 20.10.2020 20:01

Mathematics, 20.10.2020 20:01



Mathematics, 20.10.2020 20:01




English, 20.10.2020 20:01



Spanish, 20.10.2020 20:01

English, 20.10.2020 20:01