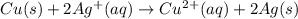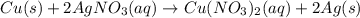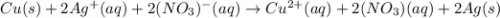Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1

Chemistry, 23.06.2019 08:20
At which temperature would a reaction with ah= -220 kj/mol and as=-0.05 kj/(mol-k) be spontaneous?
Answers: 2
You know the right answer?
Copper metal is placed in a solution of silver nitrate. Write out the net ionic of the net equation...
Questions

Chemistry, 28.10.2020 06:50

Mathematics, 28.10.2020 06:50

English, 28.10.2020 06:50


History, 28.10.2020 06:50

Mathematics, 28.10.2020 06:50

English, 28.10.2020 06:50

Mathematics, 28.10.2020 06:50




Business, 28.10.2020 07:00

History, 28.10.2020 07:00

Mathematics, 28.10.2020 07:00

Spanish, 28.10.2020 07:00

Mathematics, 28.10.2020 07:00

History, 28.10.2020 07:00



Mathematics, 28.10.2020 07:00