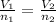Chemistry, 22.05.2021 01:00 Walkman2092
A container is filled with helium gas. It has a volume of 2.25 liters and contains 9.00 moles of helium. How many moles of helium could be held in a 1.85 liter container at the same temperature and pressure?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Joseph has hypothesized that sound travels in waves. if he were following the scientific method, what should he do next? a. ask a question. b. test the hypothesis. c. study the results. d. tell other scientists about his hypothesis.
Answers: 1

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
A container is filled with helium gas. It has a volume of 2.25 liters and contains 9.00 moles of hel...
Questions








Social Studies, 08.04.2021 17:00



Biology, 08.04.2021 17:00

Mathematics, 08.04.2021 17:00


History, 08.04.2021 17:00


Mathematics, 08.04.2021 17:00


Mathematics, 08.04.2021 17:00


Mathematics, 08.04.2021 17:00

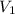 = 2.25 L,
= 2.25 L, 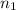 = 9.0 mol
= 9.0 mol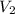 = 1.85 L,
= 1.85 L, 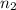 = ?
= ?