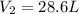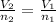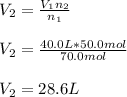Chemistry, 21.05.2021 21:30 jaylinzavala
A 40.0 liter gas tank contains 70.0 moles of hydrogen gas. What would be the volume of the tank which could hold 50.0 moles of hydrogen gas at the same temperature and pressure

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2


Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

You know the right answer?
A 40.0 liter gas tank contains 70.0 moles of hydrogen gas. What would be the volume of the tank whic...
Questions

Social Studies, 30.07.2019 02:50



Biology, 30.07.2019 02:50

Biology, 30.07.2019 02:50

History, 30.07.2019 02:50

French, 30.07.2019 02:50

Mathematics, 30.07.2019 02:50





Mathematics, 30.07.2019 02:50


Mathematics, 30.07.2019 02:50





Mathematics, 30.07.2019 02:50