
Chemistry, 21.05.2021 20:10 dari122223
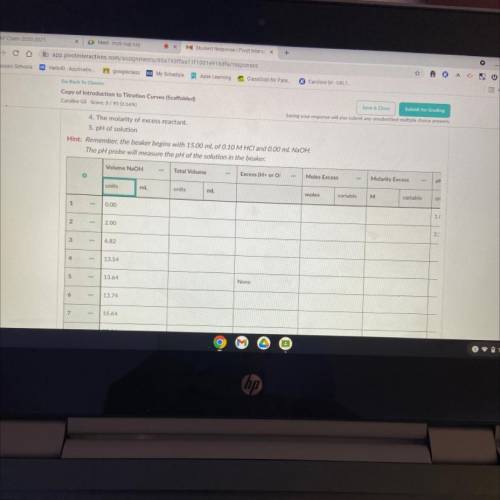
6. Now that you've predicted the equivalence point, let's predict several other points on the titration curve.
Perform calculations to predict the pH at the following points in the titration.
• 0.00 ml NaOH added
• 2.00 ml NaOH
• Half of the equivalence point volume
• 0.10 ml before the equivalence point
• Equivalence Point
• 0.10 mL after equivalence point
• 2.00 mL after the equivalence point
• 10.00 mL after equivalence point

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1
You know the right answer?
6. Now that you've predicted the equivalence point, let's predict several other points on the titrat...
Questions

English, 25.01.2022 21:20



Mathematics, 25.01.2022 21:20



Mathematics, 25.01.2022 21:20


SAT, 25.01.2022 21:20




Biology, 25.01.2022 21:20



Mathematics, 25.01.2022 21:20


Engineering, 25.01.2022 21:20

Mathematics, 25.01.2022 21:20




