Write the formula for the for the following reaction:
Use:
...

Chemistry, 21.05.2021 20:00 nayelieangueira
Write the formula for the 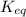 for the following reaction:
for the following reaction:
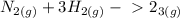
Use:
![K_{eq} =\frac{[C]^{c} [D]^d }{[A]^a[B]^b}](/tpl/images/2369/8760/65bf2.png)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3
You know the right answer?
Questions



Mathematics, 30.11.2020 22:30







Mathematics, 30.11.2020 22:30

Mathematics, 30.11.2020 22:30

Biology, 30.11.2020 22:30

Mathematics, 30.11.2020 22:30


Business, 30.11.2020 22:30


Mathematics, 30.11.2020 22:30


Biology, 30.11.2020 22:30

Physics, 30.11.2020 22:30



