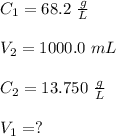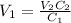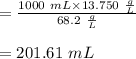Chemistry, 21.05.2021 17:50 aderahd7352
A stock solution has a concentration of 68.2 g/L. A 13.750 g/L solution is required. If you use a 1000.0 mL volumetric flask for the dilution, what volume (in ml) needs to be taken from the stock solution? Give your answer to three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1
You know the right answer?
A stock solution has a concentration of 68.2 g/L. A 13.750 g/L solution is required. If you use a 10...
Questions

Biology, 27.01.2021 18:20


Spanish, 27.01.2021 18:20

Mathematics, 27.01.2021 18:20



Mathematics, 27.01.2021 18:20

Social Studies, 27.01.2021 18:20



Law, 27.01.2021 18:20

History, 27.01.2021 18:20



Mathematics, 27.01.2021 18:20

Mathematics, 27.01.2021 18:20



Business, 27.01.2021 18:20