
Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1
You know the right answer?
What is the percent of S K2S?...
Questions

Mathematics, 21.01.2021 19:10

English, 21.01.2021 19:10

Mathematics, 21.01.2021 19:10

Mathematics, 21.01.2021 19:10

Computers and Technology, 21.01.2021 19:10



Biology, 21.01.2021 19:10


Chemistry, 21.01.2021 19:10

Chemistry, 21.01.2021 19:10

Mathematics, 21.01.2021 19:10

History, 21.01.2021 19:10



Mathematics, 21.01.2021 19:10


Business, 21.01.2021 19:10

Mathematics, 21.01.2021 19:10

Chemistry, 21.01.2021 19:10

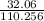 =.290777... this is your percent in decimal form, so multiply by 100 to get you percentage.
=.290777... this is your percent in decimal form, so multiply by 100 to get you percentage.

