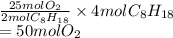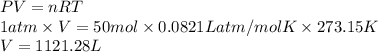Chemistry, 21.05.2021 16:30 kirstenb278
If 4.00 moles of gasoline are burned according to the chemical
reaction below, what volume of oxygen at STP is needed for complete
combustion?
2C2H18(I) + 25O2(g) → 16CO2(g) +18H2O(g)
Need done in 20 min if anyone could I help I’ll mark you as a brainiest!!

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1

Chemistry, 22.06.2019 22:00
How many moles of no2 will form when 3.3 moles of cu are reacted with excess hno3?
Answers: 3

Chemistry, 23.06.2019 00:00
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1
You know the right answer?
If 4.00 moles of gasoline are burned according to the chemical
reaction below, what volume of oxyge...
Questions

Mathematics, 03.02.2021 01:30

Geography, 03.02.2021 01:30

Business, 03.02.2021 01:30

History, 03.02.2021 01:30

Mathematics, 03.02.2021 01:30

Biology, 03.02.2021 01:30

Arts, 03.02.2021 01:30

English, 03.02.2021 01:30



Mathematics, 03.02.2021 01:30

Biology, 03.02.2021 01:30


Mathematics, 03.02.2021 01:30

English, 03.02.2021 01:30

Mathematics, 03.02.2021 01:30


Mathematics, 03.02.2021 01:30

Mathematics, 03.02.2021 01:30

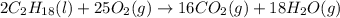
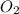 . Hence, moles of oxygen required to react with 4 moles of gasoline are calculated as follows.
. Hence, moles of oxygen required to react with 4 moles of gasoline are calculated as follows.