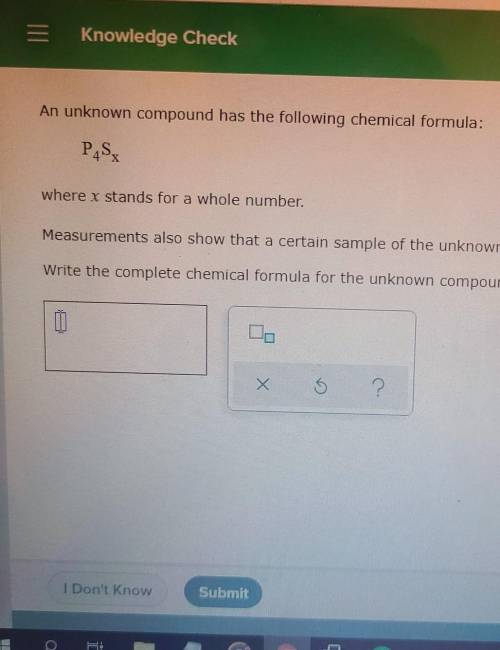
An unknown compound has the following chemical formula:
P4Sx
where x stands for a whole...

Chemistry, 21.05.2021 14:00 katherineweightman
An unknown compound has the following chemical formula:
P4Sx
where x stands for a whole number.
Measurements also show that a certain sample of the unknown compound contains 2.9 mol of sulfur and 1.94 mol of phosphorus. Write the complete chemical formula for the unknown compound.
will give brainliest :]

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3
You know the right answer?
Questions

Mathematics, 18.06.2021 18:00

Advanced Placement (AP), 18.06.2021 18:00


Computers and Technology, 18.06.2021 18:00

Arts, 18.06.2021 18:00


Computers and Technology, 18.06.2021 18:00

Mathematics, 18.06.2021 18:00

Mathematics, 18.06.2021 18:00



Mathematics, 18.06.2021 18:00



Mathematics, 18.06.2021 18:00

Biology, 18.06.2021 18:00


Physics, 18.06.2021 18:00

Mathematics, 18.06.2021 18:00

Computers and Technology, 18.06.2021 18:00



