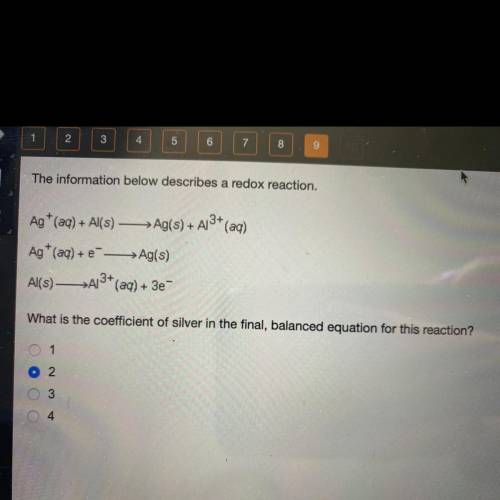
The information below describes a redox reaction.
Ag+ (aq) + Al(s) — Ag(s) + A13+ (aq)
Ag+ (a...

Chemistry, 20.05.2021 22:40 lilpeepxliltracy
The information below describes a redox reaction.
Ag+ (aq) + Al(s) — Ag(s) + A13+ (aq)
Ag+ (aq) + --> Ag(s)
Al(s)—>A13+ (aq) + 3e-
What is the coefficient of silver in the final, balanced equation for this reaction?
1
2
3
4

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:10
How is 0.00235 expressed in proper scientific notation? a. 2.35 × 10-3 b. 0.235 × 10-2 c. 2.35 d. 2.35 × 103
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3
You know the right answer?
Questions

English, 21.09.2019 14:50




History, 21.09.2019 14:50

History, 21.09.2019 14:50






Biology, 21.09.2019 14:50

Physics, 21.09.2019 14:50



Social Studies, 21.09.2019 14:50

History, 21.09.2019 14:50

Health, 21.09.2019 14:50




