
Chemistry, 20.05.2021 21:20 tylonhouse362
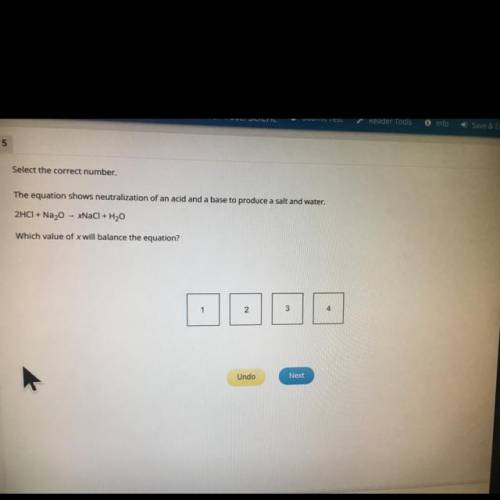
Select the correct number.
The equation shows neutralization of an acid and a base to produce a salt and water.
2HCI + Na20 - NaCl + H2O
Which value of x will balance the equation?
2.
3
4
Reset
Next

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 13:00
One significant difference between an ionic bond, where electrons are taken from one atom and added to another atom, and a covalent or metallic bond, where electrons are shared is
Answers: 2

Chemistry, 22.06.2019 00:00
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2
You know the right answer?
Select the correct number.
The equation shows neutralization of an acid and a base to produce a sal...
Questions





Mathematics, 14.01.2021 02:10









Mathematics, 14.01.2021 02:10

Mathematics, 14.01.2021 02:10

Mathematics, 14.01.2021 02:10

Computers and Technology, 14.01.2021 02:10


Mathematics, 14.01.2021 02:10



