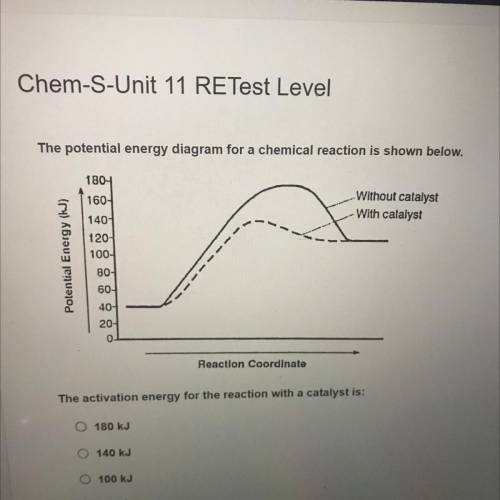
The potential energy diagram for a chemical reaction is shown below.
Without catalyst
With ca...

Chemistry, 20.05.2021 17:20 AnonymousLizard52303
The potential energy diagram for a chemical reaction is shown below.
Without catalyst
With catalyst
Potential Energy (J)
1804
160-
140
120-
100-
80-
60-
40-
20-
0
Reaction Coordinato
The activation energy for the reaction with a catalyst is:
O 180 kJ
140 kJ
100 kJ
60 kJ

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 23.06.2019 03:50
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3
You know the right answer?
Questions

Mathematics, 09.07.2019 23:30



Mathematics, 09.07.2019 23:30


English, 09.07.2019 23:30


Mathematics, 09.07.2019 23:30



History, 09.07.2019 23:30

Spanish, 09.07.2019 23:30


Mathematics, 09.07.2019 23:30


History, 09.07.2019 23:30

Mathematics, 09.07.2019 23:30


English, 09.07.2019 23:30



