
Chemistry, 20.05.2021 17:20 keilahkimbrough8
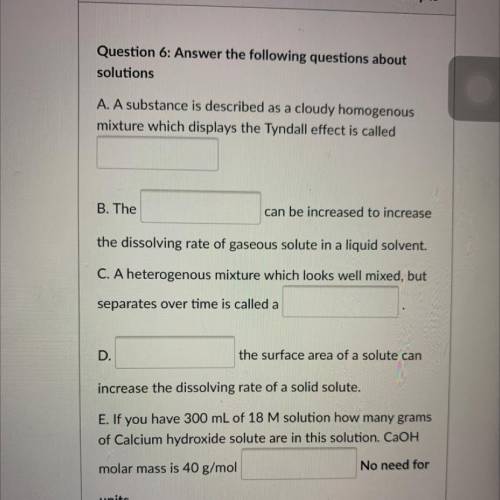
A. A substance is described as a cloudy homogenous
mixture which displays the Tyndall effect is called
B. The
can be increased to increase
the dissolving rate of gaseous solute in a liquid solvent.
C. A heterogenous mixture which looks well mixed, but
separates over time is called a
D.
the surface area of a solute can
increase the dissolving rate of a solid solute.
E. If you have 300 mL of 18 M solution how many grams
of Calcium hydroxide solute are in this solution. CaOH
No need for
molar mass is 40 g/mol
units.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 23.06.2019 09:00
A2-kg bowling ball is 1 meter off the ground on a post when it falls. just before it reaches the ground,its traveling 4.4 m/s. assuming that there is no air resistant, which statement is true a. the initial potential energy is less then the final kinetic energy b. the mechanical energy is not conserved c. the mechanical energy is conserved d. the initial potential energy is greater than the final kinetic energy
Answers: 3

Chemistry, 23.06.2019 11:20
When using the ideal gas law constant 0.0821, what unit is used for volume? a) galloonb) ouncec) milliliterd) liter
Answers: 1
You know the right answer?
A. A substance is described as a cloudy homogenous
mixture which displays the Tyndall effect is cal...
Questions

Mathematics, 23.02.2021 20:20

History, 23.02.2021 20:20

Mathematics, 23.02.2021 20:20







History, 23.02.2021 20:20


Mathematics, 23.02.2021 20:20


Mathematics, 23.02.2021 20:20


Mathematics, 23.02.2021 20:20



Mathematics, 23.02.2021 20:20

English, 23.02.2021 20:20



