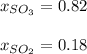Chemistry, 19.05.2021 22:00 jeancarlo1107
What is the mole fraction of each component of a gas mixture that contains sulfur trioxide at a pressure of 1.45 atm and sulfur dioxide at a pressure of .32 atm

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2
You know the right answer?
What is the mole fraction of each component of a gas mixture that contains sulfur trioxide at a pres...
Questions

Mathematics, 25.05.2021 01:00


Mathematics, 25.05.2021 01:00

History, 25.05.2021 01:00

Mathematics, 25.05.2021 01:00


Mathematics, 25.05.2021 01:00


Mathematics, 25.05.2021 01:00


English, 25.05.2021 01:00

Mathematics, 25.05.2021 01:00



History, 25.05.2021 01:00



History, 25.05.2021 01:00

Mathematics, 25.05.2021 01:00

Computers and Technology, 25.05.2021 01:00