Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x.xx ml
Answers: 1

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1
You know the right answer?
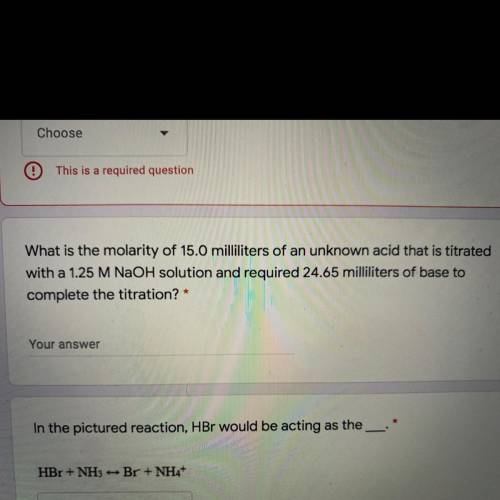
What is the molarity of 15.0 milliliters of an unknown acid that is titrated
with a 1.25 M NaOH sol...
Questions


Mathematics, 27.09.2021 22:40

World Languages, 27.09.2021 22:40





Mathematics, 27.09.2021 22:40

Mathematics, 27.09.2021 22:40


Mathematics, 27.09.2021 22:40

English, 27.09.2021 22:40


Mathematics, 27.09.2021 22:40

Mathematics, 27.09.2021 22:40

Mathematics, 27.09.2021 22:40

Mathematics, 27.09.2021 22:40



English, 27.09.2021 22:40