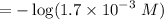Chemistry, 19.05.2021 19:10 shealwaysknows23
Calculate the concentration of H3O+ ions present in a solution of HCl that has a measured pH of 1.510 .
M
HCl, the acid in Part 1, is a strong acid. The concentration of H3O+ ions in this solution is the same as the initial concentration of the acid.
A solution of the weak acid CH3COOH with the same initial concentration of acid will have a pH that is (Higer, lower, the same) the pH of the HCl solution.
Rank the following solutions in order of increasing acidity, placing the most acidic solution at the left. (CH3COOH is approximately 1.0% ionized at this concentration.)
pH= 0.00, pH= 7.45, [HCl]= .15M, [CH3COOH]= .15M
order of acidity
most acidic > least acidic

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 11:00
Ais a mountain created from eruptions of lava, ash, rocks, and hot gases.
Answers: 1


Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3
You know the right answer?
Calculate the concentration of H3O+ ions present in a solution of HCl that has a measured pH of 1.51...
Questions

English, 13.06.2020 18:57

Mathematics, 13.06.2020 18:57



Mathematics, 13.06.2020 18:57







Mathematics, 13.06.2020 18:57



Mathematics, 13.06.2020 18:57

Mathematics, 13.06.2020 18:57




Computers and Technology, 13.06.2020 18:57

![$[H_3O^+]$](/tpl/images/1334/2671/dab07.png) is pH =
is pH = ![$- \log [H_3O^+]$](/tpl/images/1334/2671/b0a97.png)
![$[H_3O^+]=10^{-pH}$](/tpl/images/1334/2671/b447d.png)
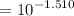
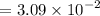
![$=- \log[0.15]$](/tpl/images/1334/2671/efddc.png)
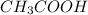 is as follows :
is as follows :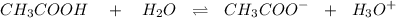
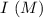 0.15 0 0
0.15 0 0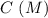 -x +x +x
-x +x +x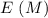 0.15 - x x x
0.15 - x x x![$K_a=\frac{[CH_3COO^-][H_3O^+]}{[CH_3COOH]}$](/tpl/images/1334/2671/7fb51.png)
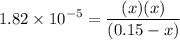
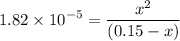
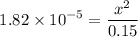
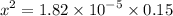
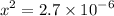
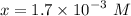
![$[H_3O^+] =x=1.7 \times 10^{-3} \ M$](/tpl/images/1334/2671/663fb.png)