Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
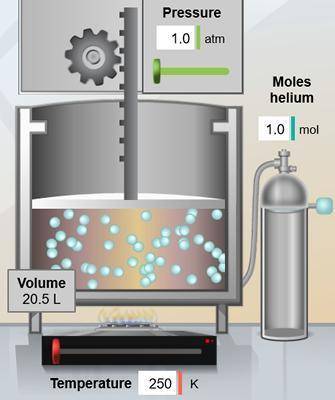
In the chamber below, the volume is 20.5 L. What would the volume be if the temperature is increased...
Questions









History, 19.09.2019 08:10

History, 19.09.2019 08:10




Biology, 19.09.2019 08:10

Chemistry, 19.09.2019 08:10