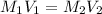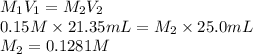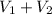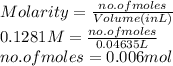Chemistry, 19.05.2021 14:00 jazminpratt0311
In an acid-base titration, a student uses 21.35 mL of 0.150 M NaOH to neutralize 25.00 mL of H2SO4. How many moles of acid are in the flask?

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 15:30
How many grams of c3h8 is needed in the reactants to produce 10.5 mil of h2o
Answers: 2

Chemistry, 23.06.2019 16:00
Henry moseley used x-ray experiments to determine the atomic number of elements. how did his discovery contribute to the development of the periodic table? a.it confirmed that elements should be arranged in strict order of increasing atomic mass. b.it led to elements with similar atomic numbers being grouped together. c.it allowed the elements to be placed in strict order of increasing atomic number. d.it showed that the way mendeleev grouped elements together was completely wrong.
Answers: 1

Chemistry, 23.06.2019 16:40
Which heterogeneous mixture contains large particles that can settle out or can be filtered? colloid compound element suspension
Answers: 1

Chemistry, 23.06.2019 19:30
There was a water with a boat and a rock in a boat. will the level of the water stay the same or higher or lower if u remove the rock from the boat and put it under water? ?
Answers: 3
You know the right answer?
In an acid-base titration, a student uses 21.35 mL of 0.150 M NaOH to neutralize 25.00 mL of H2SO4....
Questions

Mathematics, 28.05.2020 20:04




Mathematics, 28.05.2020 20:04

History, 28.05.2020 20:04





Health, 28.05.2020 20:04

Computers and Technology, 28.05.2020 20:04


Mathematics, 28.05.2020 20:04

Mathematics, 28.05.2020 20:04

English, 28.05.2020 20:04


English, 28.05.2020 20:04


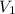 = 21.35 mL,
= 21.35 mL, 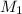 = 0.150 M
= 0.150 M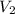 = 25.0 mL,
= 25.0 mL, 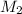 = ?
= ?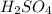 is as follows.
is as follows.