2
3 K 4
KM
5
67
2a. A 51 g piece of metal is heated to 100°C and placed in...

Chemistry, 19.05.2021 01:00 morenoozzie
2
3 K 4
KM
5
67
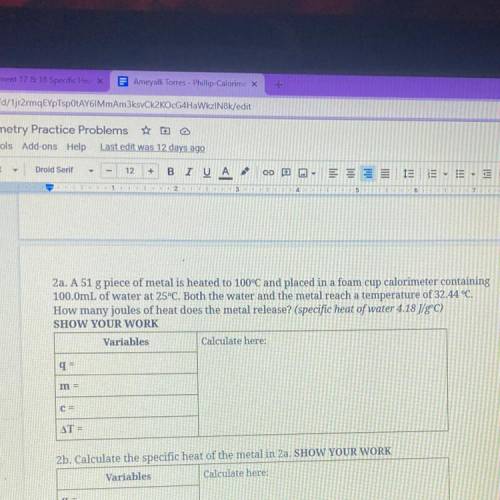
2a. A 51 g piece of metal is heated to 100°C and placed in a foam cup calorimeter containing
100.0mL of water at 25°C. Both the water and the metal reach a temperature of 32.44 °C.
How many joules of heat does the metal release? (specific heat of water 4.18 J/gºC)
SHOW YOUR WORK
Variables
Calculate here:
q=
m =
C=
AT =

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1


Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

You know the right answer?
Questions

Biology, 21.12.2019 22:31

Mathematics, 21.12.2019 22:31

English, 21.12.2019 22:31


History, 21.12.2019 22:31

Biology, 21.12.2019 22:31

Mathematics, 21.12.2019 22:31

Physics, 21.12.2019 22:31

History, 21.12.2019 22:31





Mathematics, 21.12.2019 22:31

Mathematics, 21.12.2019 22:31



History, 21.12.2019 22:31




