3
1 point
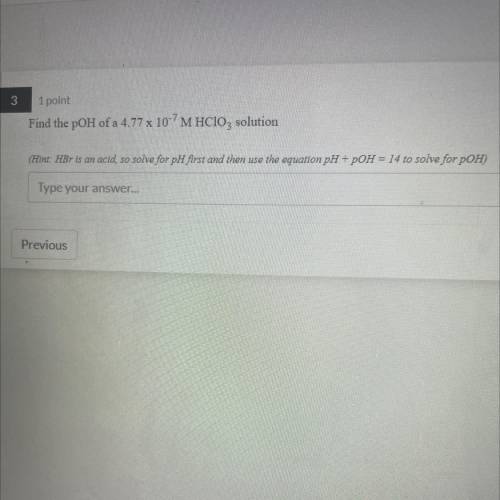
Find the pOH of a 4.77 x 10-7 M HClO3 solution
(Hint: HBr is an acid, so solv...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
Questions



Mathematics, 01.08.2019 20:00









Mathematics, 01.08.2019 20:00

Biology, 01.08.2019 20:00



Mathematics, 01.08.2019 20:00


Arts, 01.08.2019 20:00

Social Studies, 01.08.2019 20:00