
Chemistry, 18.05.2021 21:10 marahsenno
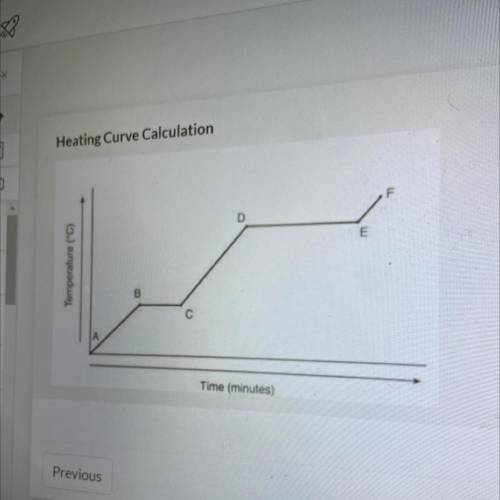
Referencing the figure to the left, how much energy in J would be
needed to change 59 g of ice at -22.75 °C into steam at 128.65 °C
Important numbers/equations:
q = Cpm: AT
Specific Heat of Water (liquid) = 4.18 J/go°C
Specific Heat of Water (solid) = 2.06 J/g•°C
Specific Heat of Water (gas) = 2.01 J/g °C
Molar Heat of Fusion of water: 6009 J/mol
Molar Heat of Vaporization for water: 40790 J/mol

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1


Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3
You know the right answer?
Referencing the figure to the left, how much energy in J would be
needed to change 59 g of ice at -...
Questions

Mathematics, 23.05.2021 19:10

Mathematics, 23.05.2021 19:10

Social Studies, 23.05.2021 19:10


Mathematics, 23.05.2021 19:10

Mathematics, 23.05.2021 19:10

Mathematics, 23.05.2021 19:10

English, 23.05.2021 19:10

History, 23.05.2021 19:10



Social Studies, 23.05.2021 19:10






Chemistry, 23.05.2021 19:10





