
Chemistry, 18.05.2021 20:30 Nismo3501037
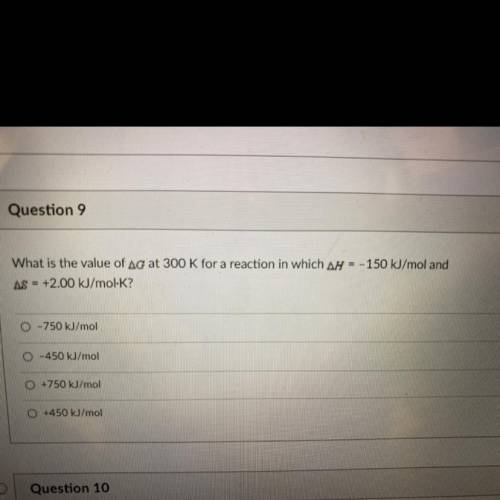
What is the value of Ag at 300 K for a reaction in which ar = - 150 kJ/mol and AS = +2.00 kJ/mol-K?

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 13:30
Explain the impact that changing the temperature has on a system in a state of dynamic equilibrium. what will happen when the temperature of an exothermic reaction mixture at equilibrium is increased?
Answers: 3

Chemistry, 23.06.2019 13:30
How does water evaporating from a glass show that matter is made up of particles? a. the heat energy from the air causes the glass to fill up with water particles. b. the liquid water particles turn into water vapor that spreads in the air. c. the particles of the glass dissolve in water and cause it to evaporate. d. the tiny particles of the glass evaporate and seem to disappear.
Answers: 2

You know the right answer?
What is the value of Ag at 300 K for a reaction in which ar = - 150 kJ/mol and
AS = +2.00 kJ/mol-K?...
Questions


Mathematics, 02.10.2019 19:10




History, 02.10.2019 19:10


Business, 02.10.2019 19:10

Computers and Technology, 02.10.2019 19:10


Mathematics, 02.10.2019 19:10


Engineering, 02.10.2019 19:10










