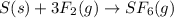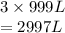Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2
You know the right answer?
What volume in liters of fluorine gas is needed to form 999 L of sulfur hexafluoride gas if the foll...
Questions

History, 08.02.2022 01:00



Mathematics, 08.02.2022 01:00

History, 08.02.2022 01:00

Biology, 08.02.2022 01:00


Mathematics, 08.02.2022 01:00



Mathematics, 08.02.2022 01:00



English, 08.02.2022 01:00

Spanish, 08.02.2022 01:00

Biology, 08.02.2022 01:00

English, 08.02.2022 01:00

English, 08.02.2022 01:00