
Chemistry, 18.05.2021 07:50 zuleromanos
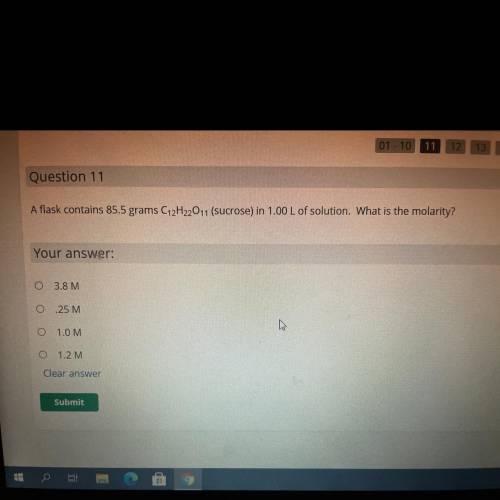
A flask contains 85.5 grams C12H2011 (sucrose) in 1.00 L of solution. What is the molarit
Your answer.
3.8 M
25 M
10M
1.2M

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1


You know the right answer?
A flask contains 85.5 grams C12H2011 (sucrose) in 1.00 L of solution. What is the molarit
Your answ...
Questions

Social Studies, 03.02.2021 22:40

Biology, 03.02.2021 22:40



Health, 03.02.2021 22:40

English, 03.02.2021 22:40


Mathematics, 03.02.2021 22:40

Mathematics, 03.02.2021 22:40

Mathematics, 03.02.2021 22:40

Mathematics, 03.02.2021 22:40



History, 03.02.2021 22:40



Physics, 03.02.2021 22:40

Physics, 03.02.2021 22:40

Arts, 03.02.2021 22:40

Mathematics, 03.02.2021 22:40



