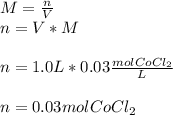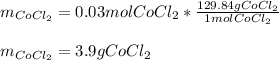Chemistry, 18.05.2021 07:10 blazenrais
Describe the process of making 1.0 L of a 0.03 M solution of cobalt (II) chloride from the solid state. Be sure to include all instrumentation and glassware.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

You know the right answer?
Describe the process of making 1.0 L of a 0.03 M solution of cobalt (II) chloride from the solid sta...
Questions


Mathematics, 09.09.2021 14:00

Mathematics, 09.09.2021 14:00

English, 09.09.2021 14:00

Mathematics, 09.09.2021 14:00




Biology, 09.09.2021 14:00


Biology, 09.09.2021 14:00




Mathematics, 09.09.2021 14:00

SAT, 09.09.2021 14:00

Medicine, 09.09.2021 14:00

Mathematics, 09.09.2021 14:00

Business, 09.09.2021 14:00