0 0
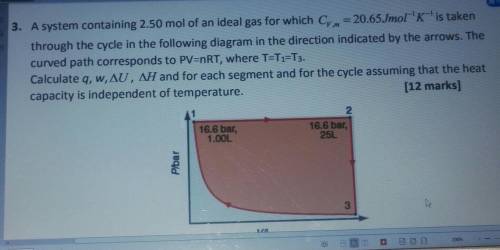
3. A system containing 2.50 mol of an ideal gas for which Cy. m = 20.65Jmol-'K-'is taken
t...

Chemistry, 17.05.2021 23:10 pablohc200021
0 0
3. A system containing 2.50 mol of an ideal gas for which Cy. m = 20.65Jmol-'K-'is taken
through the cycle in the following diagram in the direction indicated by the arrows. The
curved path corresponds to PV=nRT, where T=T1=T3.
Calculate q, w, AU, AH and for each segment and for the cycle assuming that the heat
capacity is independent of temperature.
(12 marks]
2.
16.6 bar,
1.00L
16.6 bar,
25L
Plbar
یا
3

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2
You know the right answer?
Questions



Chemistry, 07.12.2019 22:31


History, 07.12.2019 22:31

Biology, 07.12.2019 22:31

Mathematics, 07.12.2019 22:31

Mathematics, 07.12.2019 22:31

Health, 07.12.2019 22:31


English, 07.12.2019 22:31

Mathematics, 07.12.2019 22:31

Physics, 07.12.2019 22:31



Geography, 07.12.2019 22:31


Mathematics, 07.12.2019 22:31

History, 07.12.2019 22:31



