
Chemistry, 17.05.2021 20:00 christi1175
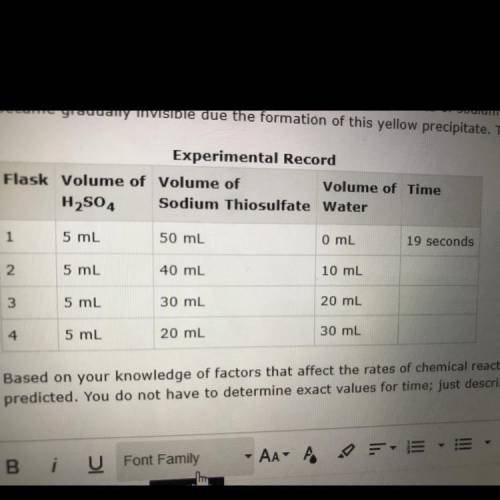
In an experiment, sulfuric acid reacted with different volumes of sodium thiosulfate in water. A yellow precipitate was formed during the reaction. A cross drawn at the base of each flask became gradually invisible due the formation of this yellow precipitate. The time taken for the cross to become invisible was recorded. A partial record of the experiment is shown. Based on your knowledge of factors that affect the rates of chemical reactions, predict the trend in the last column of the experimental record. Use complete sentences to explain the trend you predicted. You do not have to determine exact values for time; just describe the trend you would expect (increase or decrease) and why it occurs.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1


Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1
You know the right answer?
In an experiment, sulfuric acid reacted with different volumes of sodium thiosulfate in water. A yel...
Questions

Spanish, 03.06.2021 22:20

Mathematics, 03.06.2021 22:20

Mathematics, 03.06.2021 22:20

Mathematics, 03.06.2021 22:20

Chemistry, 03.06.2021 22:20




Mathematics, 03.06.2021 22:20

Mathematics, 03.06.2021 22:20

Mathematics, 03.06.2021 22:20

Mathematics, 03.06.2021 22:20

Mathematics, 03.06.2021 22:20


Mathematics, 03.06.2021 22:20


Mathematics, 03.06.2021 22:20



Physics, 03.06.2021 22:20



