Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1

Chemistry, 23.06.2019 02:30
Asubstance is held in an open container. its particles move past one another at random speeds but do not leave the container. heat is removed from the system, and the particles slow down. when enough heat is removed, the particles no longer have enough speed to overcome the weak attractive forces between them. when this happens, the substance enters its solid state. the process described above is known as .
Answers: 3
You know the right answer?
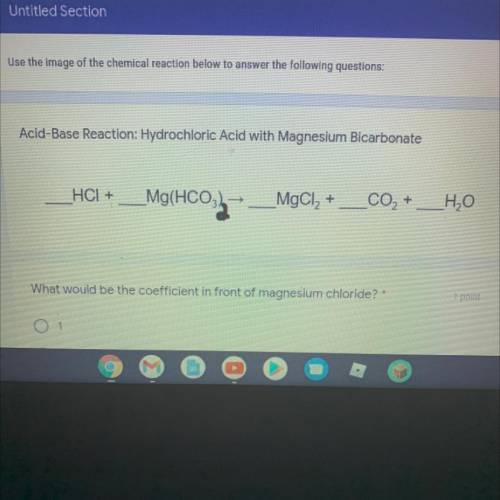
Acid-Base Reaction: Hydrochloric Acid with Magnesium Bicarbonate
__HCI + _Mg(HCO3)2 —> _MgCl2 +...
Questions





Computers and Technology, 26.08.2020 03:01



Mathematics, 26.08.2020 03:01



Mathematics, 26.08.2020 03:01

Biology, 26.08.2020 03:01

Mathematics, 26.08.2020 03:01

Mathematics, 26.08.2020 03:01

Mathematics, 26.08.2020 03:01

Mathematics, 26.08.2020 03:01


Mathematics, 26.08.2020 03:01