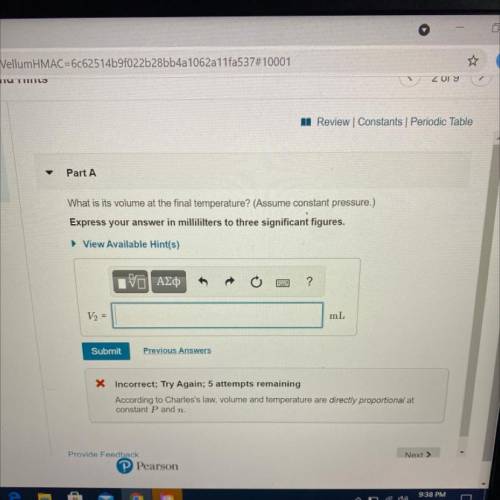
A 48.5 mL sample of gas in a cylinder is warmed
from 18°C to 92°C.
What is its volume a...


Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
In the analysis of hair and fiber samples, which does a compound comparison microscope allow for that a conventional compound microscope does not? a. simultaneous observation b. polarization c. fluorescence d. higher magnification
Answers: 2

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 23:00
Need asap question 1 minerals are organic compounds. true false question 2 what vitamin can be found in foods like oranges, grapefruits, and broccoli? a. vitamin a b. vitamin k c.vitamin c d. vitamin d question 3 what are minerals? a. chemical elements that are needed for body processes. b. organic compounds that the body needs in small amounts to function properly. c. small molecules used to build proteins. d. an organic compound that is insoluble in water and includes fats. question 4 how many types of vitamins does the human body need? a. 15 b. 11 c. 13 d. 17 question 5 vitamins are a good source of energy. true false
Answers: 1

Chemistry, 23.06.2019 05:40
Why is any chemical reaction always balanced? give reasons and explain the easiest way to solve the balancing problems in chemical equations with stoichiometric coefficients upto 20 as hit and trial doesn't always work. give full reasoning
Answers: 1
You know the right answer?
Questions


Chemistry, 14.12.2020 18:10

Chemistry, 14.12.2020 18:10

Social Studies, 14.12.2020 18:10






Engineering, 14.12.2020 18:10


SAT, 14.12.2020 18:10



Computers and Technology, 14.12.2020 18:10

French, 14.12.2020 18:10


Biology, 14.12.2020 18:10

English, 14.12.2020 18:10

Mathematics, 14.12.2020 18:10