
Chemistry, 16.05.2021 02:20 bunnybuge4
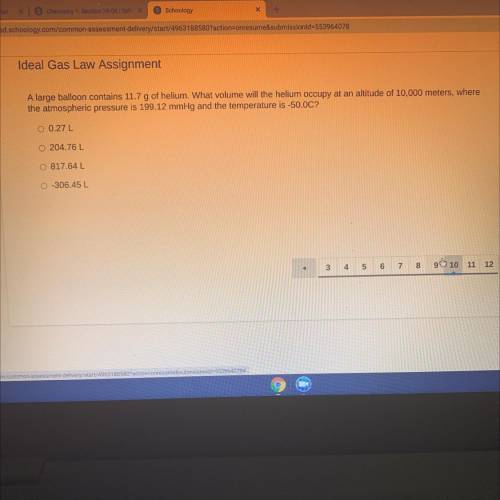
A large balloon contains 11.7 g of helium. What volume will the helium occupy at an altitude of 10 000 m, where the atmospheric pressure is 199.12 mmHg and the temperature is -50.0 C

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
A large balloon contains 11.7 g of helium. What volume will the helium occupy at an altitude of 10 0...
Questions




Biology, 22.04.2021 02:00


Mathematics, 22.04.2021 02:00


Mathematics, 22.04.2021 02:00


Mathematics, 22.04.2021 02:00


Mathematics, 22.04.2021 02:00



Computers and Technology, 22.04.2021 02:00




Mathematics, 22.04.2021 02:00

Mathematics, 22.04.2021 02:00




