
Chemistry, 16.05.2021 01:30 carlshiabrown
(12 points multiple choice )A chemist pours two chemicals into a beaker and observes their reactions. Using the data table determine if the reaction is
a. Exothermic and spontaneous
b. Endothermic and spontaneous
c. Exothermic and not spontaneous
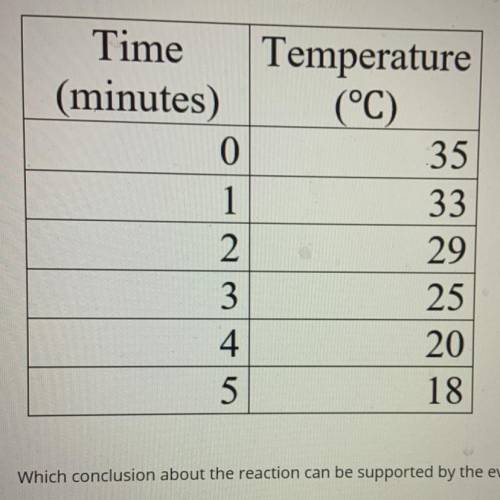

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1


Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1
You know the right answer?
(12 points multiple choice )A chemist pours two chemicals into a beaker and observes their reactions...
Questions

Mathematics, 27.05.2020 04:58


Mathematics, 27.05.2020 04:58


Social Studies, 27.05.2020 04:58





Mathematics, 27.05.2020 04:58

Mathematics, 27.05.2020 04:58


Mathematics, 27.05.2020 04:58


English, 27.05.2020 04:58


Mathematics, 27.05.2020 04:58


Mathematics, 27.05.2020 04:58





