
Chemistry, 15.05.2021 22:50 haileyw123
Who is really good with chemistry and can help me with some questions/equations? I also have more i really don't get this subject
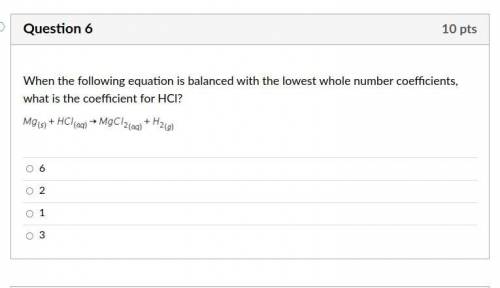

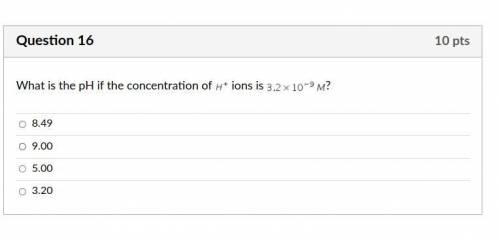
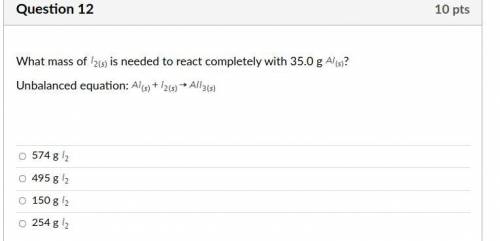
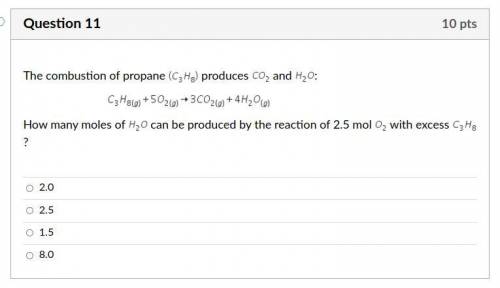

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2
You know the right answer?
Who is really good with chemistry and can help me with some questions/equations? I also have more i...
Questions

Mathematics, 05.03.2021 04:10

English, 05.03.2021 04:10



Mathematics, 05.03.2021 04:10

Mathematics, 05.03.2021 04:10



Mathematics, 05.03.2021 04:10

History, 05.03.2021 04:10

History, 05.03.2021 04:10

English, 05.03.2021 04:10







Mathematics, 05.03.2021 04:10

English, 05.03.2021 04:10



