
Chemistry, 14.05.2021 23:20 saltyimps3
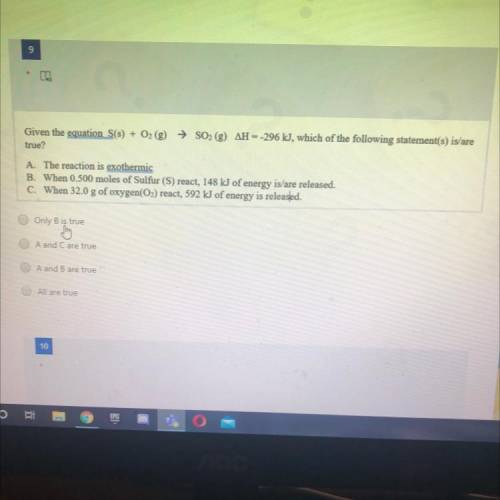
Given the equation S(s) + O2 (g) → SO2 (g) AH --296 kJ, which of the following statement(s) is/are
true?
A. The reaction is exothermic
B. When 0.500 moles of Sulfur (S) react, 148 kJ of energy is/are released.
C. When 32.0 g of oxygen(O2) react, 592 kJ of energy is released.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
You know the right answer?
Given the equation S(s) + O2 (g) → SO2 (g) AH --296 kJ, which of the following statement(s) is/are...
Questions


Mathematics, 26.03.2021 03:10




Mathematics, 26.03.2021 03:10


Mathematics, 26.03.2021 03:10


Computers and Technology, 26.03.2021 03:10

Social Studies, 26.03.2021 03:10

Mathematics, 26.03.2021 03:10

Mathematics, 26.03.2021 03:10



Mathematics, 26.03.2021 03:10


Mathematics, 26.03.2021 03:10

History, 26.03.2021 03:10



