
Chemistry, 14.05.2021 23:20 andrew8228
R-134a is contained in a frictionless piston-cylinder device. The initial temperaure of the mixture is 39.37 oC. Over an hour 400 kJ of thermal energy is transferred to the roon which is maintained at a constant temperature of 22 oC. The condensation process is internally reversible. Determine the total entropy generation during this thermal energy process

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2
You know the right answer?
R-134a is contained in a frictionless piston-cylinder device. The initial temperaure of the mixture...
Questions

Mathematics, 03.12.2021 06:50

History, 03.12.2021 06:50



Mathematics, 03.12.2021 07:00








History, 03.12.2021 07:00

Business, 03.12.2021 07:00


Mathematics, 03.12.2021 07:00

English, 03.12.2021 07:00



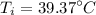
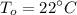
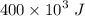
![$\Delta S =\left[\frac{-Q}{T_i}+ \frac{+Q}{T_i}\right]$](/tpl/images/1325/3377/40417.png)
 K.
K.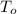 K.
K. ![$\Delta S =\left[\frac{-400\times 10^3}{312.3}+ \frac{400\times 10^3}{295}\right]$](/tpl/images/1325/3377/8fa96.png)


