
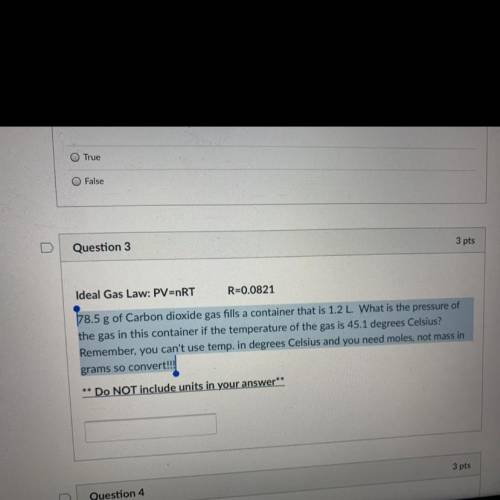
PLEASSE HELP! 78.5 g of Carbon dioxide gas fills a container that is 1.2 L. What is the pressure of the gas in this container if the temperature of the gas is 45.1 degrees Celsius? Remember, you can't use temp. in degrees Celsius and you need moles, not mass in grams so convert!!!

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 07:30
The scheme below is from a series of reactions that are part of a synthesis of vitamin a. answer the following questions with reference to this scheme. (i) what is "reagent a"? (ii) draw a step-by-step mechanism which explains the formation of compound c from compound b (iii) which reagents would you use to form compound e from compounds c and d (reagents b and c)? for each reagent suggested above in (ii) explain the role of the reagent in the reaction to (iv) form compound e. you may wish to do this by drawing a mechanism. 1. addition of reagent a но reagent a 2. н,о" thо oh нон-с compound a. compound b. compound c .ch-оh 1. reagent b "сно 2. reagent c сh oh compound e. compound d.
Answers: 2


Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
PLEASSE HELP!
78.5 g of Carbon dioxide gas fills a container that is 1.2 L. What is the pressure of...
Questions


Social Studies, 23.01.2020 22:31



Mathematics, 23.01.2020 22:31












Computers and Technology, 23.01.2020 22:31





