Chemistry, 14.05.2021 18:40 shanicar33500
The specific heat capacity of liquid water is 4.18 J/g oC. Calculate the quantity of energy required to heat 1.50 g of water from 26.5oC to 83.7oC. (Ignore significant figures for this problem.)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2

You know the right answer?
The specific heat capacity of liquid water is 4.18 J/g oC. Calculate the quantity of energy required...
Questions



English, 12.01.2021 01:00

Social Studies, 12.01.2021 01:00


Mathematics, 12.01.2021 01:00


Mathematics, 12.01.2021 01:00

History, 12.01.2021 01:00

Mathematics, 12.01.2021 01:00



Arts, 12.01.2021 01:00

Mathematics, 12.01.2021 01:00



Mathematics, 12.01.2021 01:00

Mathematics, 12.01.2021 01:00

History, 12.01.2021 01:00

Mathematics, 12.01.2021 01:00

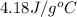
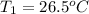
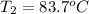
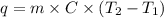
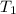 = initial temperature
= initial temperature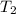 = final temperature
= final temperature