
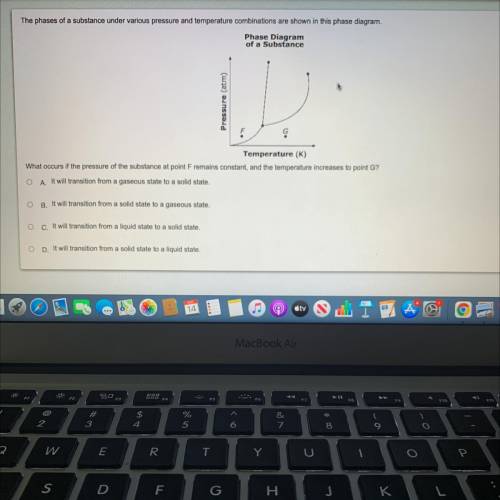
The phases of a substance under various pressure and temperature combinations are shown in this phase
What occurs if the pressure of the substance at point Fremains constant, and the temperature increases to point G?
A It will transition from a gaseous state to a solid state.
B. It will transition from a solid state to a gaseous state.
c. It will transition from a liquid state to a solid state.
D. It will transition from a solid state to a liquid state.
tv

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
150.0 grams of asf3 were reacted with 180.0 g of ccl4 to produce ascl3 and ccl2f2. if the actual yield of ccl2f2 was 127 g, what is the percent yield?
Answers: 2

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1
You know the right answer?
The phases of a substance under various pressure and temperature combinations are shown in this phas...
Questions



Geography, 26.07.2019 09:30


Social Studies, 26.07.2019 09:30



Health, 26.07.2019 09:30









History, 26.07.2019 09:30





