ALL In Learning
Show Summary
Previous
Next
→
CHEM QUIZ 4 7 ON 5 14 21
...

Chemistry, 14.05.2021 18:00 shartman22
ALL In Learning
Show Summary
Previous
Next
→
CHEM QUIZ 4 7 ON 5 14 21
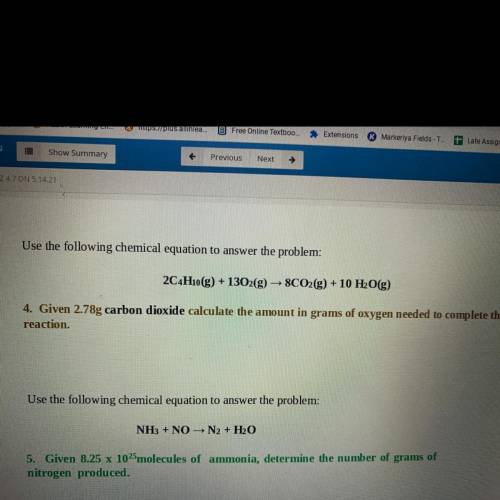
Use the following chemical equation to answer the problem:
2C4H10(g) + 1302(g) → 8CO2(g) + 10 H2O(g)
4. Given 2.78g carbon dioxide calculate the amount in grams of oxygen needed to complete the
reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1
You know the right answer?
Questions

Physics, 18.09.2021 14:00

English, 18.09.2021 14:00

Physics, 18.09.2021 14:00


History, 18.09.2021 14:00



Social Studies, 18.09.2021 14:00

Mathematics, 18.09.2021 14:00





Mathematics, 18.09.2021 14:00

Biology, 18.09.2021 14:00



Spanish, 18.09.2021 14:00





